Strategy & Operations – Kind Consumer
Early-hire at disruptive inhalation technology start-up. Developed product from drawing-board to market-ready consumer product with Medical Regulatory Approval. Raised over £30m in equity financing and partnered with FTSE10 business for commercialisation deal worth over £100m.
Early Member of Disruptive Pharma Startup
From Concept to UK Market Launch
Involved in the end-to-end product development process, taking Voke from initial concept to full regulatory approval and market launch in the UK.
Gaining UK Regulatory Approval with MHRA
Collaborated with the Chief Medical Officer and technical teams to secure MHRA approval by demonstrating Voke’s quality, safety, and efficacy.
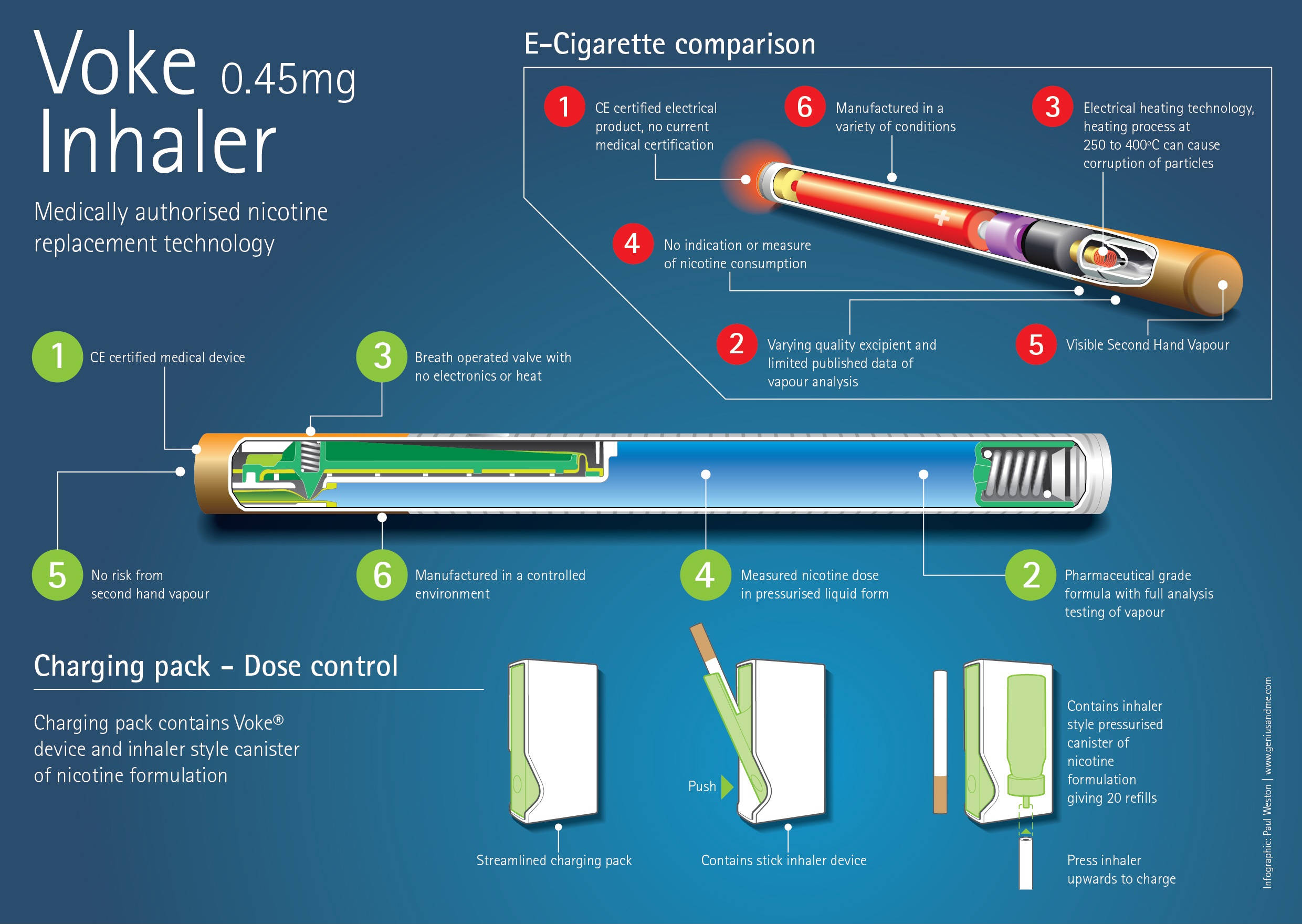
Commercial Partnership with FTSE10 Company
Member of the negotiating team which secured a £100m commercial partnership with British American Tobacco (BAT) to bring Voke inhalation technology to market, creating a brand new category.

Securing £30m in Funding
Contributed in securing over £30m in funding from Angel investors, Venture Capital, and corporate sources, driving the technology from concept to market.
Worked Alongside a High-Profile Board
Worked on a board comprising former CEOs, Private Equity experts, marketing veterans, and medical professionals.